Affinity Plus™ DNA & RNA Oligonucleotides
Oligos incorporating locked nucleic acids for enhanced stability and nuclease resistance
Affinity Plus DNA & RNA Oligonucleotides are custom, single-stranded, and duplexed sequences that contain 1−20 locked nucleic acid nucleotides. Incorporation of the locked nucleic acid nucleotides offers increased target specificity and oligo stability [1]. Refer to our Locked nucleic acids technology page to see the structure of these modified bases and to learn about the many applications they can facilitate.
Ordering
- Cost-efficient, reliable oligos for a variety of hybridization-based applications
- Helps you manage sequence melting temperature, resulting in greater hybridization specificity
- Locked nucleic acids can improve stability and exonuclease resistance (in vitro and in vivo)
Affinity Plus Single-Stranded DNA
Shipped dry, or resuspended to your specifications.
Affinity Plus Duplexed DNA
2 oligos, annealed and delivered in a single tube. Shipped dry.
The following annealing fees will be applied to each duplex ordered:
Affinity Plus Single-Stranded RNA
Shipped dry.
Affinity Plus Duplexed RNA
2 oligos, annealed and delivered in a single tube. Shipped dry.
Annealing fees will be applied to duplexed oligos (per duplex), as follows:
Affinity Plus Single-Stranded DNA
Shipped dry, or resuspended to your specifications. A minimum of 24 and 96 oligos required for 96- and 384-well plates, respectively.
Affinity Plus Duplexed DNA
1 oligo pair per well, annealed. Shipped dry. A minimum of 24 and 96 oligo duplexes are required for 96- and 384-well plates, respectively.
For ordering inquiries, please contact us.
Affinity Plus Single-Stranded RNA
Shipped dry or resuspended to your specifications. A minimum of 24 or 96 oligos required for 96- or 384-well plates, respectively.
For ordering inquiries, please contact us.
Product details
Affinity Plus DNA & RNA Oligonucleotides are locked nucleic acids containing single- and double-stranded sequences. With the Oligo Entry ordering tool, you can design the sequence you require with 1−20 locked nucleic acid nucleotides to suit your research needs.
Every Affinity Plus DNA & RNA Oligo you receive is deprotected and desalted to remove small molecule impurities. In addition, the identity of your oligos will be confirmed via proprietary ESI-mass spectrometry methods* and quantified by UV spectrophotometry to provide accurate yield measurements.
*With the exception of mixed base oligos, which could potentially represent multiple sequences therefore cannot accurately be evaluated by ESl mass spectrometry.
Using the Oligo Entry tool
To enter individual Affinity Plus nucleotides, insert a "+" before the base (+A, +C, +G, +T).
Product data
Affinity Plus oligonucleotides increase stability
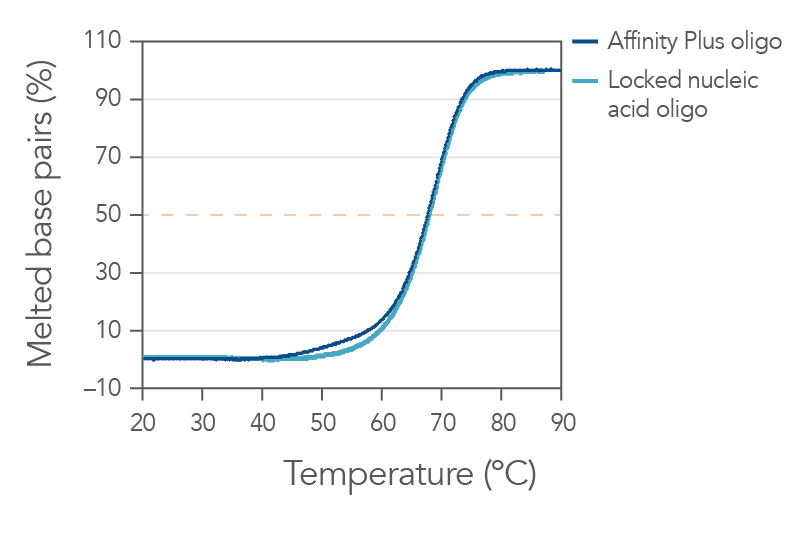
Affinity Plus Oligonucleotides provide identical annealing properties as other manufacturers' locked nucleic acid sequences (Figure 1). Oligonucleotides that have locked nucleic acids have enhanced stability, increased melting temperatures, and higher nuclease resistance, relative to unmodified oligonucleotides.
Figure 1. Sequences containing Affinity Plus oligonucleotides show identical annealing properties to locked nucleic acid sequences from another vendor. The percentages of duplex melted base pairs are plotted as a function of temperature. The 15-mer sequence, GGTCCT+T+A+CTTGGTG, was synthesized with either Affinity Plus or other locked nucleic acid modifications incorporated at the +T, +A, and +C sites. These oligos were mixed with the complementary DNA strand (1 µM each strand) in 1 M Na+ buffer (pH 7). Melt curves were performed as described in Owczarzy, et al. [2]. The plots of duplex melted base pairs were averaged from at least 7 heating and cooling melting curves. Melting temperature was measured to be 67.7 ± 0.3 °C for the Affinity Plus oligo and 67.9 ± 0.3°C for the other locked nucleic acid oligo. Free energy of duplex hybridization at 37°C was determined to be -18.1± 0.9 kcal/mol for the Affinity Plus oligo and -18.7 ± 0.9 kcal/mol for the other locked nucleic acid oligo. These results illustrate the identical annealing properties of both sources of locked nucleic acid sequences.
Target identification in qPCR and SNP assays
The Affinity Plus sequences can be used as probes, for example, in qPCR and genotyping assays. Relative to unmodified oligonucleotides, the Affinity Plus RNA and DNA oligos have an increased affinity for targeted regions. Further the incorporation of Affinity Plus into these oligos makes them more stable than unmodified oligonucleotides [1]. This increased stability also makes it possible to use shorter probe designs, which can be helpful when target regions that are of a limited size [1].
For these applications, you can obtain probes as Affinity Plus qPCR probes. These Affinity Plus qPCR probes provide the same precise amplification as other locked nucleic acid probes and show more rapid target amplification over unmodified probes (see Figure 2 on the Product data tab of the Affinity. Plus qPCR Probes products page).
Resources
Frequently asked questions
What is the difference between the locked nucleic acids in Affinity Plus™ qPCR Probes and PrimeTime™ LNA® qPCR Probes?
There are no structural differences between the locked nucleic acids used in Affinity Plus and PrimeTime LNA* qPCR Probes. Functional data can be found on the Affinity Plus qPCR Probes page. While these modified probes have identical chemical structures and physical properties, Affinity Plus qPCR Probes provide a better value.
Learn more about Affinity Plus qPCR Probes.
*LNA is a registered trademark of Qiagen.
How do I convert my yield from nanomoles to ODs?
Calculating this conversion requires you to know the extinction coefficient for your sequence. With this, you can easily convert from your nanomole amount to ODs. To determine the extinction coefficient, you can analyze your sequence using IDT's free OligoAnalyzer™ Tool. Results from this analysis will also provide you with nmol/OD260 and µg/OD260 values.
Are locked nucleic acids available for custom DNA and RNA oligos?
Yes, locked nucleic acids are available as Affinity Plus™ DNA & RNA Oligonucleotides, which can be designed to contain 1−20 locked nucleic acid bases.
To include an Affinity Plus base in your sequence, simply place “+” in front of the base, e.g., +A+C+G+T.
Learn more about Affinity Plus DNA & RNA Oligonucleotides.
Will you synthesize aptamers?
Yes. If you can provide the aptamer sequence(s), you can order directly from the IDT website.
To order online click on Custom DNA oligos, or if your sequence contains RNA bases, Custom RNA oligos.
Enter your desired scale, sequence, and purification.If you are ordering aptamers with a fixed sequence, IDT recommends HPLC or PAGE purification. For aptamer libraries containing random bases, IDT recommends standard desalt.
Why should I consider purification of my oligos?
Purification removes truncated products and other synthesis impurities. Our experience has shown that purifying an oligonucleotide that will be used in demanding applications saves both time and money in the long run. We recommend considering additional purification for any oligonucleotide that will be used for an application other than routine PCR, qPCR, or Sanger DNA sequencing.
As a general rule, IDT also recommends that any oligonucleotide longer than 40 bases should receive further purification. For questions, please contact us.
Why do my primers have deleted 3' bases in the amplified fragment when I use a proofreading enzyme for PCR?
Proofreading polymerases (Pfu, Diamond Taq®, etc) have been shown to remove 3' bases from PCR primers [1].
If you experience this problem, you can protect your primer from degradation by adding one or more phosphorothioate bonds to the 3' end of your primer.
Also make sure to add the enzyme last, after the addition of dNTPs, in your PCR to minimize the risk of this issue taking place.
Reference
1. Skerra A. Phosphorothioate primers improve the amplification of DNA sequences by DNA polymerases with proofreading activity. Nucleic Acids Res. 1992;20(14):3551-3554.
Why are my oligos a yellow/brown color?
Customers who are new to working with large amounts of material are sometimes surprised when they see a yellow or brown discoloration to their oligos. If you have experienced this, do not worry. Color variation is normal in custom synthesis, especially as concentration increases. Importantly, IDT does not expect this to affect oligo function in our customers' experimental applications.

The image above depicts normal color variation as seen in oligo pools which are formulated to the same concentration (200 nmol oligos in 15 mL solution). In general, modified oligos at larger scales are more likely to display the variation seen above, while small-scale unmodified oligos (e.g., 25 nmol) tend to remain colorless in solution.
When calculating the Tm of my oligo, should I only use the bases which are complementary to my target, or should I also include any sticky overhangs on the primer?
To determine the relative Tm of primers with non-complementary overhangs, only the complementary region should be taken into account.
You can obtain the Tm using the free, online OligoAnalyzer™ Tool.
What molar concentrations of oligonucleotide and salts are used to calculate the Tm reported on specification sheets?
The Tm value reported on our spec sheets uses a 25 µM oligonucleotide and a 50 mM salt concentration. It does not take into account dNTP or Mg2+ concentrations.
To get an accurate Tm for your specific application, all of these concentrations should be entered and adjusted to match the reaction conditions you plan to use.
The free IDT OligoAnalyzer™ calculates Tm for the concentrations you input.
What is the standard desalting procedure IDT uses for newly synthesized oligos, and what additional purification options do I have?
IDT uses a proprietary desalting technique that removes some truncation products and small organic contaminants from the synthesized oligonucleotide preparation. Removal of n-1mers produced during synthesis requires additional PAGE or HPLC purification.
PAGE is recommended for unmodified oligos >80−100 bases, while HPLC is the preferred method for oligos modified with either fluorescent dyes or attachment chemistries.
What is the stability of RNA vs. DNA?
RNA is inherently less stable than DNA due to its chemical structure. Additionally, RNases are more prevalent in standard laboratory conditions than DNases.
As even the slightest exposure to RNase can impact RNA stability, IDT has not performed rigorous long term stability studies for RNA.
What is the difference between scale and yield and why don’t I receive that full amount designated by the scale I order?
Oligo synthesis is accomplished through a series of steps, including coupling of individual bases, cleaving the oligo from the solid support, desalting, and if requested, purification of the oligo by HPLC or PAGE. No chemical reaction occurs with 100% efficiency, and, thus, each of these steps will incur a loss of final yield, which varies from specific sequence synthesis to synthesis.
Due to this variation, IDT custom oligos are ordered according to the amount of starting material used for the synthesis, referred to as the scale. While we cannot predict the actual final yield, we do guarantee a specific minimum yield for each oligo based on its sequence, starting scale, and the typical yield obtained under those specified conditions.
If you would like to know the minimum guaranteed yield for a specific oligo, simply add it to your Shopping Cart. The Shopping Cart provides you with the guaranteed minimum yield. If it is insufficient or excessive for your needs, simply edit the scale.
Please contact us if you have any questions about the yield you have received.
What is the best way to purify PCR products?
For most applications, it is best to purify PCR products by gel electrophoresis.
This simple method not only purifies the final PCR product but can be a valuable troubleshooting tool as well since nonspecific PCR products, primer-dimer products, negative amplification, and other elements can easily be identified by gel analysis.
What does OD260 stand for?
The heterocyclic ring structures in DNA and RNA absorb light with a maximum absorbance near 260 nanometers (nm). An OD260, or optical density 260, is defined as the amount of light at a 260 nm wavelength which will be absorbed by an oligo resuspended in 1 mL water and the concentration is read in a 1 cm quartz cuvette.
This method of measurement is considered the most accurate means of assessing the amount of oligonucleotide present following synthesis. The relationship between measured OD260, molar extinction coefficient (ε260), and oligonucleotide concentration is given as: OD260 = ε260 x concentration.
What conditions should I use to HPLC purify my oligos?
IDT uses our own proprietary HPLC method to purify oligos in-house. A published set of conditions for an RP-HPLC protocol is:
- Hamilton PRP-1 Reverse Phase column 250 x 10 mm
- Buffer A = 100% ACN (acetonitrile)
- Buffer B = ACN/0.1 M TEAA pH 7.3
- Run at 4 mL/min over 30 min from 95–60% A
- Monitor at 260/297 nm
What are the length restrictions for your DNA oligos?
Standard DNA oligos are synthesized up to 100 bases, while our high fidelity Ultramer™ Oligonucleotides can provide single-stranded DNA up to 200 bases.
Beyond 200 bases, we offer our new gBlocks™ Gene Fragments which are double-stranded DNA constructs available from 126 bp up to 2000 bp.
What are self- and homodimers, and how do I select primers to avoid these problems?
DNA and RNA oligonucleotides can form adverse secondary structures.
Self-dimers (also called homodimers) occur when some portion of an oligonucleotide is complementary to itself, resulting in an oligonucleotide molecule that can hybridize to another oligonucleotide molecule of the exact same sequence.
We recommend checking these interactions using the IDT OligoAnalyzer™ Tool, which is available for free in the online SciTools™ Web Tools.
What are aptamers and why are they growing in popularity?
Aptamers are RNA or DNA oligonucleotides (or peptides) that, through their 3-dimensional structures, bind to target molecules with a high level of precision. They are often identified using a technique such as SELEX (Systematic Evolution of Ligands by EXponential enrichment), where oligos with increased affinity and specificity to the target molecules are isolated from the sequence pool after several rounds of selection.
These molecules can serve as a substitute for antibodies used to identify a specific target. They have similar affinities as antibodies for their targets and provide several advantages, including greater stability, easier large-scale production, low immunogenicity, and the ability to target molecules with low antigenicity. Like antibodies, aptamers have a broad range of research applications. The sequences are often modified to enhance stability during in vitro and in vivo use.
Should I only be concerned about sodium ions when calculating melting temperature (Tm)?
Any charge will play a role in the Tm value for an oligonucleotide in a reaction. Thus, sodium, magnesium, and potassium concentrations each contribute to this calculation.
The IDT OligoAnalyzer™ Tool will calculate Tm for you, taking into account the presence of specific ions in the reaction buffer.
Should I do anything to prep my tube of oligo before resuspending?
We recommend briefly centrifuging your tubes of dried oligo prior to opening them.
This will ensure that the oligo pellet is at the bottom of the tube and will not be lost when you open the cap. After adding non-DEPC treated water or buffer (e.g., TE or IDTE), briefly vortex the tube, but do not centrifuge it.
References
- Kurreck J, Wyszko E, Gillen C, Erdmann VA. Design of antisense oligonucleotides stabilized by locked nucleic acids. Nucleic Acids Res. 2002;30(9):1911-8. doi:10.1093/nar/30.9.1911
- Owczarzy R, You Y, Moreira BG, et al. Effects of sodium ions on DNA duplex oligomers: improved predictions of melting temperatures. Biochemistry. 2004;43(12):3537-54. doi:10.1021/bi034621r