Introduction
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) is a method used to quantify nucleic acids in samples [1]. However, this approach often requires samples to undergo nucleic acid extraction which can be laborious, time consuming, and can result in the loss of precious samples. Experiments can be hindered further when reactions are inefficient due to a poorly designed master mix. Here, we highlight the newly formulated
PrimeTime One-Step 4X Broad-Range Master Mix. This Master Mix has been specifically designed by IDT researchers for one-step RT-qPCR reactions. The 4X mixture of the mutant hot-start reverse transcriptase, mutant hot-start DNA polymerase, dNTPs and buffer provides high-quality performance for analysis of RNA. In developing this Master Mix, IDT researchers tested over 35,000 buffer components and selected the ideal mixture, generating a high-quality, flexible, and reliable master mix. The included enhancer solution was specifically designed to overcome inhibitors and improve amplification when using crude samples.
PrimeTime One-Step 4X Broad-Range Master Mix
Amplify RNA from crude or purified nasopharyngeal and saliva samples
The
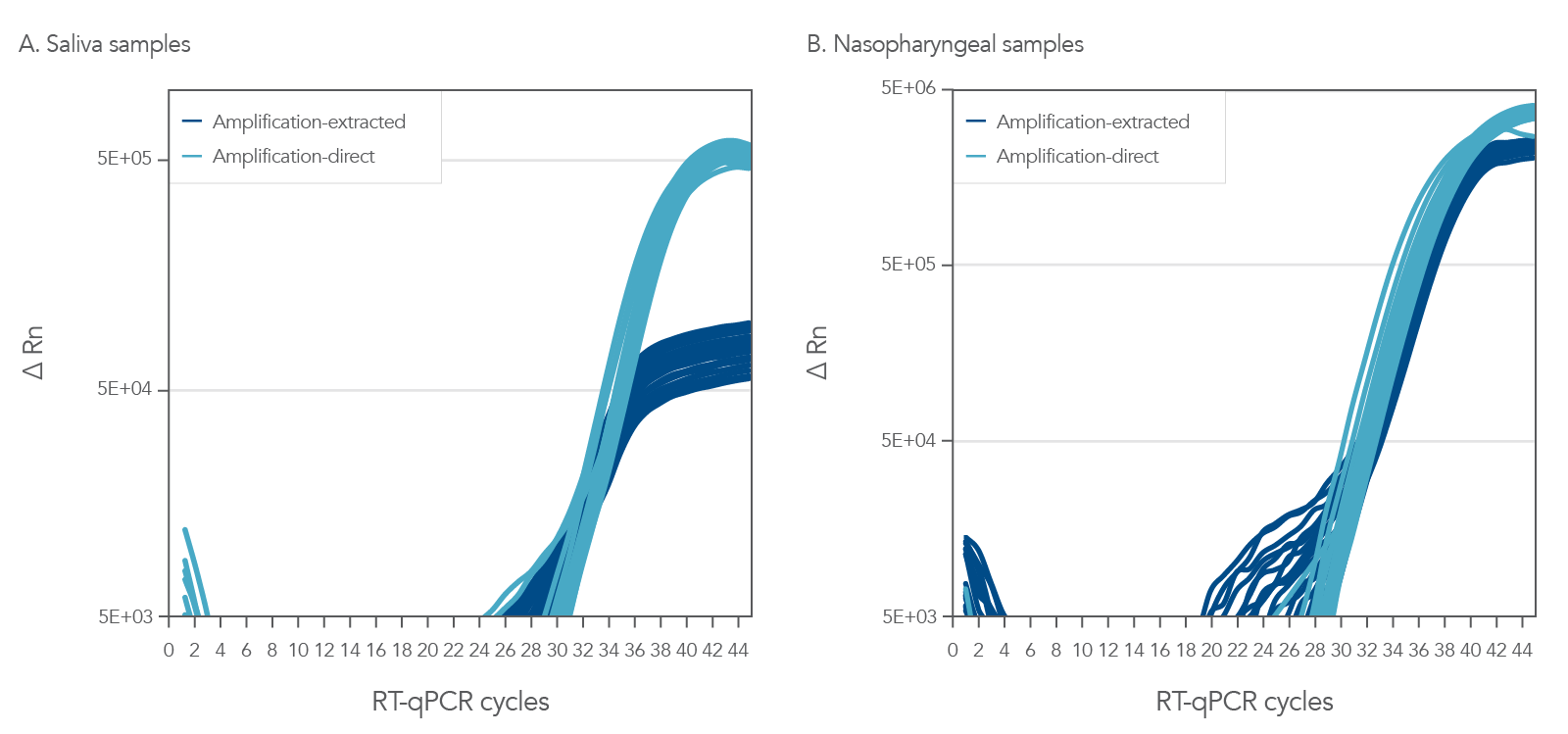
PrimeTime One-Step 4X Broad-Range Master Mix can consistently and reliable amplify from either crude or purified nasopharyngeal (NP) and saliva samples (Figure 1). In this experiment, we amplified both NP and saliva samples that were incubated in viral transport medium (VTM) with ~10 copies of Accuplex SARS-CoV-2 reference material. These samples were then used crude or subject to an RNA extraction with the Applied Biosystems™ MagMax™ II Viral/Pathogen Isolation Kit (MVP II). The results shown in Figure 1 clearly illustrate the utility of this Master Mix to give you the flexibility to use either a direct amplification method or a purified sample method for amplification.
Consistent and reliable multiplex amplification reaction
The
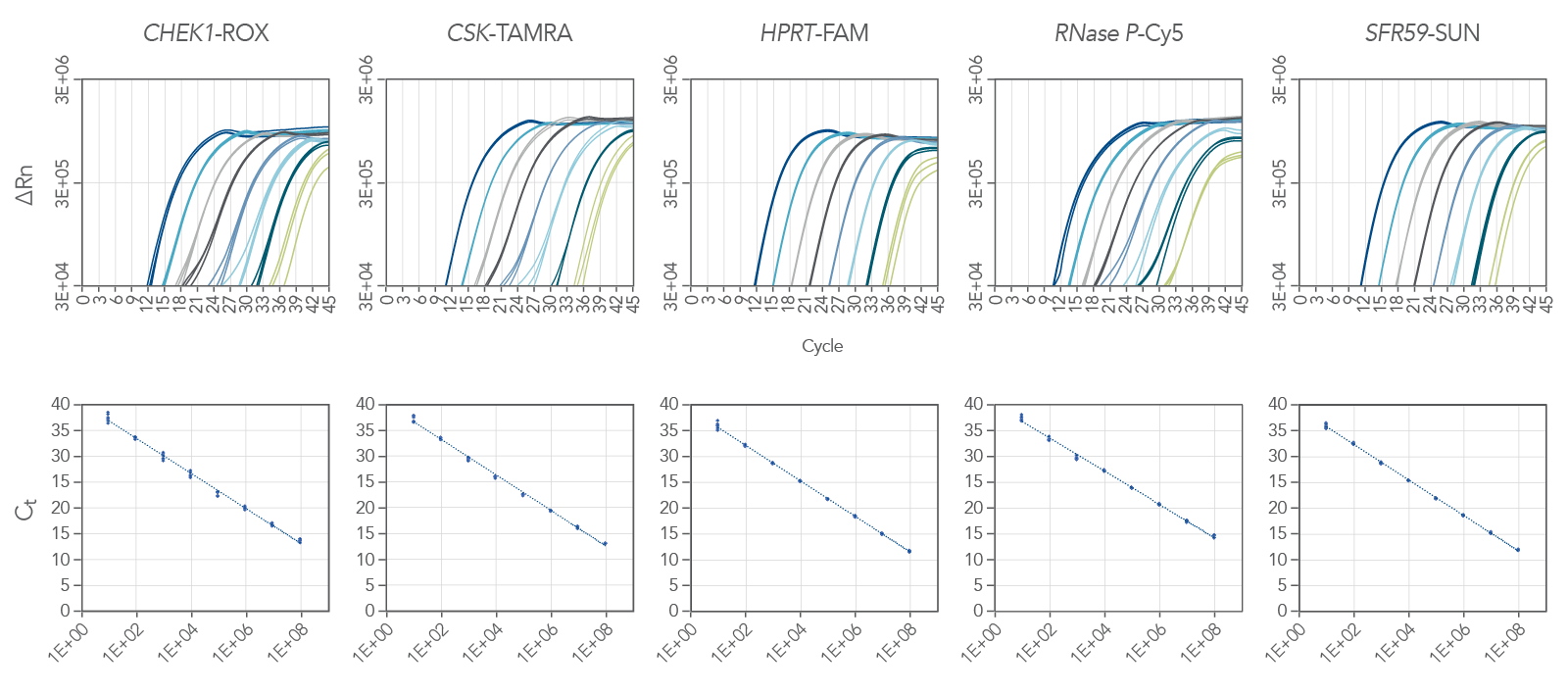
PrimeTime One-Step 4X Broad-Range Master Mix is ideal for multiplex qPCR. Figure 2 shows amplification of a 5-plex reaction, demonstrating the master mix’s ability to handle these types of challenging experiments. Here we amplified 5 targets:
CHEK1 (ROX),
CSK (Tamara),
HPRT (FAM),
RNAse P (Cy5) and
SFRS9 (SUN). We amplified all the targets over 8 logs of RNA template with a reaction efficiency between 94.9% and 105.1% (Figure 2).
Lower Ct values and higher end-point fluorescence than other vendors
The
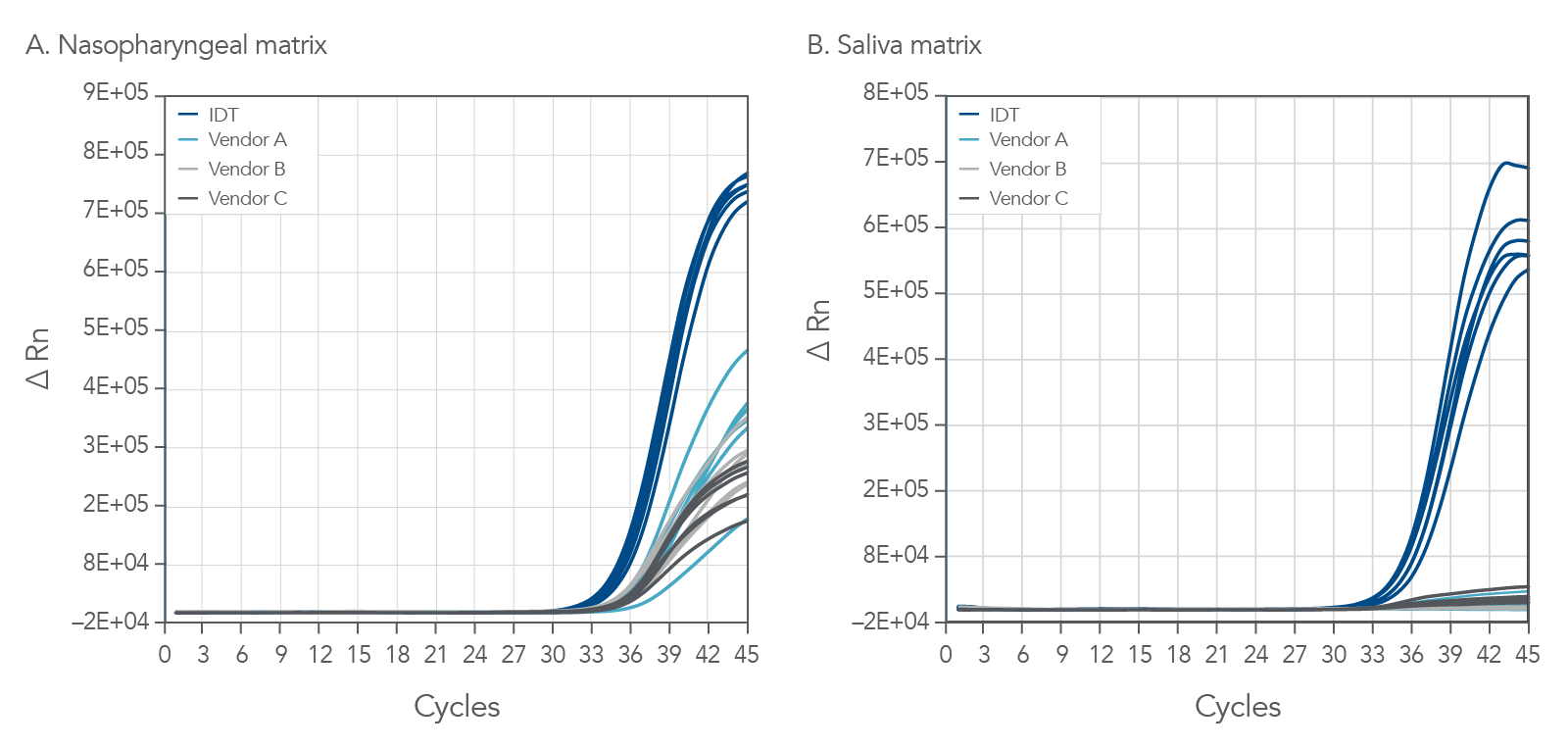
PrimeTime One-Step 4X Broad-Range Master Mix was challenged against other comparable commercially available master mixes. As seen in Figure 3, the PrimeTime One-Step 4X Broad-Range Master Mix shows both lower Ct and higher end-point fluorescence in comparison to other commercially available master mixes running the same assays. Assays for Influenza A (InfA, shown), Influenza B (InfB), SARS-CoV-2 and RNAse P were run with direct amplification from samples containing 30 copies of viral specimens in both human NP and human saliva samples. The PrimeTime One-Step 4X Broad-Range Master Mix reliably detected low copy numbers with direct amplification on unpurified samples and provided confidence in data analysis with high end-point fluorescence, particularly at later cycles.
Lowering the limit of identification
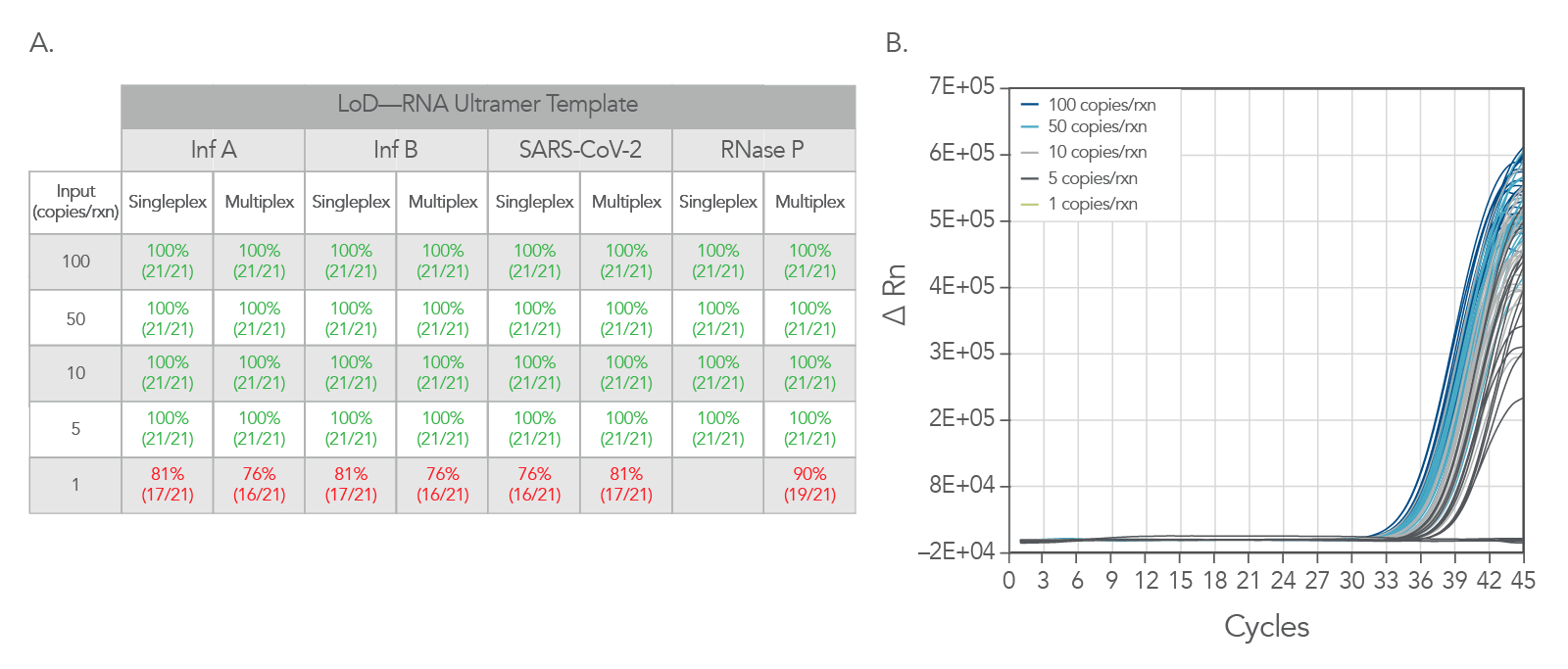
Limit of identification data was generated using
Ultramers™ RNA Oligonucleotides as a template. Ultramers were diluted from 100 copies/rxn down to 1 copy per reaction. As can be seen Figure 4, the PrimeTime One-Step 4X Broad-Range Master Mix shows excellent results down to 5 copies per reaction.
Conclusions
A reliable, flexible, and dependable master mix is critical for RT-qPCR approaches that generates powerful quantitative data. The
PrimeTime One-Step 4X Broad-Range Master Mix can be used with samples that have not been extracted. Further the data presented above illustrates the utility of this master mix in multiplexed reactions as well as its ability to accelerate your RT-qPCR method by generating lower Ct values and higher endpoint fluorescence than other vendors.
Author(s)
Courtney Thomas, PhD, Scientific Writer, IDT